Composting plays an important role in returning nitrogen to the soil. Nitrogen is an organic element critical for plant growth – so this nutrient recovery is a significant benefit compared to landfilling or thermal conversion processes that may release the nitrogen to atmosphere.
However, the process of converting nitrogen into a usable form for plants through composting is complex and requires time. The following describes some of the reactions that can take place, and how compost process conditions affect this nitrogen mineralization process:
The figure below, adapted from the Compost Handbook, provides a visual of the potential pathways for nitrogen:
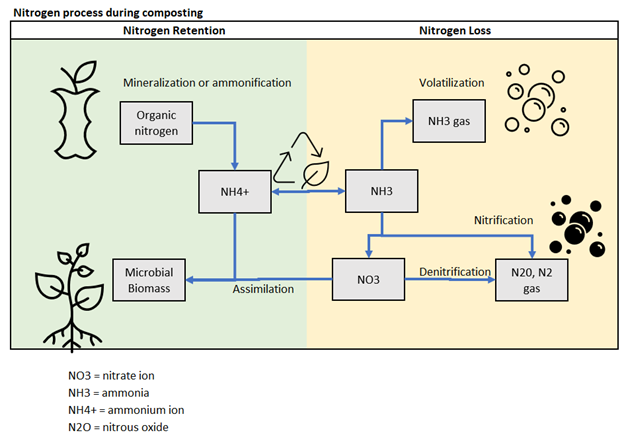
Ammonia (NH3) and ammonium (NH4+) are both forms of nitrogen compounds. The key difference between them lies in their chemical composition and behavior in different environments.
Ammonia is a highly volatile gas with a strong, pungent odor. Ammonia is produced naturally by the breakdown of nitrogen-containing organic matter, such as urine, animal waste, or decaying plant material. Ammonium refers to the positively charged ion NH4+. It is the result of ammonia reacting with water (H2O) and accepting a proton (H+), forming the ammonium ion. This occurs under acidic conditions. Ammonium is a more stable form of nitrogen, especially in environments with lower pH levels. It is often found in aqueous solutions or bound to solid particles. In a composting application, both ammonia and ammonium play important roles. Initially, during the decomposition of organic matter, ammonia tends to be released as a byproduct. However, as the composting process continues, ammonia can transform into ammonium under the right temperature and pH conditions.
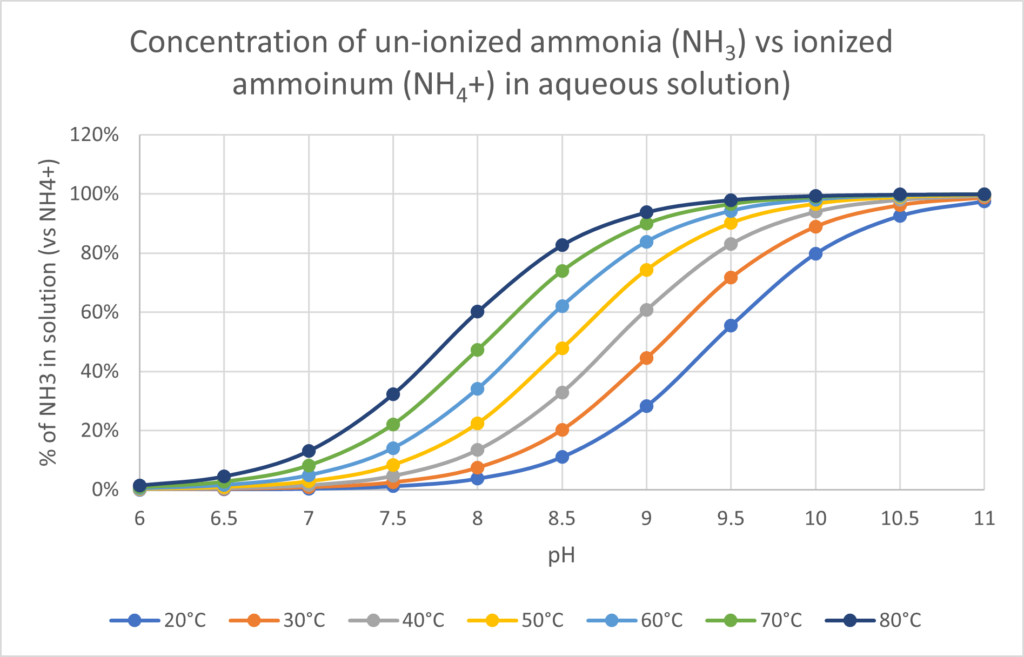
In the ammonification process, the ratio of Ammonia (NH3) and Ammonium (NH4+) depends on the pH and temperature. Hot, alkaline conditions lead to more ammonia formation. Cooler temperatures and neutral or acidic pH leads to more ammonium formation.
Especially for composting, high ammonia can lead to ammonia toxicity and inhibit the biological process. This inhibition can often lead to formation of other odorous compounds (such as reduced sulfurs, which is often an issue with biosolids composting).
Nitrate (NO3-) and nitrite (NO2-) are also forms of nitrogen compounds, but they are different from ammonia and ammonium. Nitrate and nitrite are oxidized forms of nitrogen that are typically produced during the later stages of composting, particularly under aerobic (oxygen-rich) conditions. Nitrate is the most oxidized form, while nitrite is an intermediate product in the process of nitrification. In composting, the conversion of ammonia and ammonium to nitrate and nitrite is a crucial step called nitrification. Nitrification is carried out by specific bacteria known as nitrifying bacteria. These bacteria convert ammonium into nitrite through a process called ammonium oxidation, and then further oxidize the nitrite into nitrate. This conversion is important because nitrate is a highly available form of nitrogen that plants can readily utilize for growth.
Several process conditions can affect these nitrogen conversion processes during composting:
As always, managing the compost nitrogen process requires control of the process conditions. The operator must verify the feedstocks are near best management practice, and the compost aeration system must be designed with adequate cooling capacity. With some effort you can optimize the nitrogen conversion and retention process during composting and produce a nutrient-rich compost that can benefit plants and soil health.
Sources:
Emerson, Russo, Lund, Thurston. “ Aqueous Ammonia Equilibrium Calculations: Effect of pH and Temperature”. Department of Chemistry, Montana State University. August 1975. Pp. 2379-2383
Rynk, Robert. “The Composting Handbook”. Academic Press. 2022.